When you hear the phrase "the acid bath murderer," it probably brings to mind a rather unsettling picture, doesn't it? That, is that, a pretty intense image, and it often sparks curiosity about the chemical substance at the heart of such a concept. We're talking about acids, of course, and what they actually are, from a scientific point of view. It's almost like peeling back the layers of a very complex idea to get to the basic building blocks of chemistry.
So, a lot of people might have a general idea of what an acid is, perhaps from school or just common talk. You might think of something that eats through things, or maybe something that just tastes really, really sour. But, as a matter of fact, the scientific definition goes a bit deeper than just those common perceptions. It's less about the dramatic effect and more about the fundamental properties that make these substances behave the way they do.
Anyway, in this discussion, we're going to pull back the curtain on acids, exploring what chemists really mean when they talk about them. We'll look at their characteristics, how they interact with other things, and where you might actually find them in your everyday surroundings. It's about getting a solid grasp on the chemical facts, which can sometimes be quite different from the stories we hear or the images that pop into our minds.
- Osinttechnical Twitter
- Cinna Twitter
- Oprah Winfrey Arrested
- Celeb Eggplant Twitter
- Charlotte Sins Twitter
Table of Contents
- What is an Acid, Anyway?
- How Do Chemists Define Acids?
- Is That Acid Strong or Weak?
- Where Do We Find Acids in Daily Life?
- What Happens When Acids Meet Bases?
- What About pH and Litmus Paper?
- Can Acids React with Metals?
- Why Are Acids So Variable?
What is an Acid, Anyway?
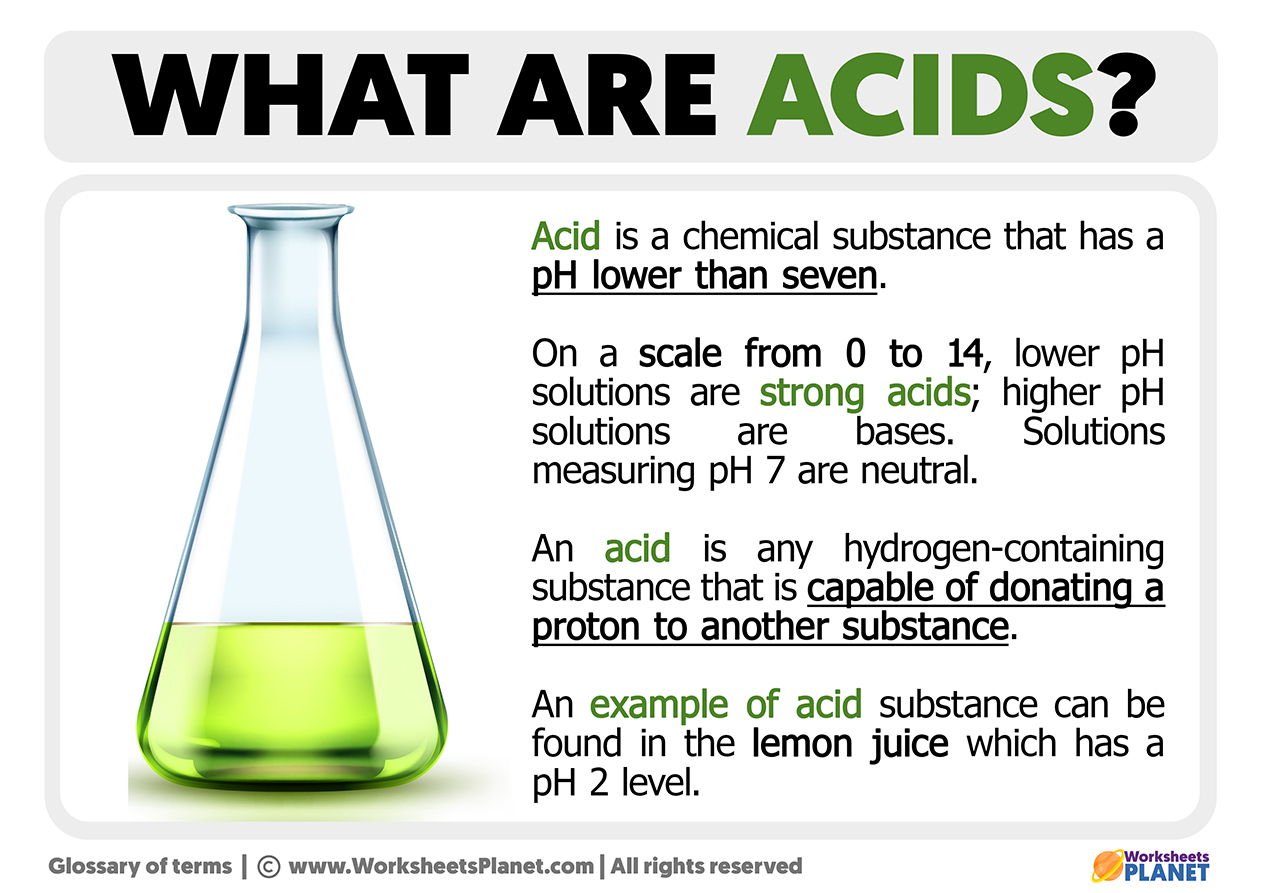
So, if you're thinking about what an acid truly is, especially in a water solution, one of the first things that comes to mind for many people is its taste. A lot of acids, in their diluted forms, can have a rather sharp, sour taste. Think about the tang of a lemon or the bite of vinegar; those are good examples of this particular characteristic. It's a pretty distinctive sensation that helps us identify some common acids in our daily experiences, just a little bit.
Then, there's the classic litmus paper test. This is a simple, yet very telling, way to figure out if something is an acid. If you take a piece of blue litmus paper and dip it into an acidic solution, you'll see a pretty quick change. The paper turns a bright red. This color shift is a clear sign that you're dealing with an acidic substance, and it's a fundamental property that chemists use to classify these materials. You know, it's a straightforward visual cue.
The Sour Truth - Understanding the Acid Bath Murderer's Core Chemical
Beyond taste and color changes, acids also have a way of interacting with certain metals. When an acid meets particular metals, a chemical reaction happens, and this reaction can actually produce hydrogen gas. You might see bubbles forming, which is the hydrogen escaping from the solution. This ability to react and release hydrogen is another important characteristic that helps us define what an acid does, more or less, in a chemical setting. It's a pretty dynamic interaction.
- Sheridan Love Twitter
- Twitter I Have The Same Shirt
- Taeguide Twitter
- Ianandmariah Twitter
- Hand Job Twitter
And then, there's the way acids behave when they encounter what we call "bases." Bases are, in a way, the chemical opposite of acids. When an acid and a base get together, they react in a process called neutralization. This reaction typically creates two new things: water and a type of compound known as a salt. It's a fundamental chemical dance, you know, where the acid gives up some parts and the base accepts them, leading to a new, often more stable, arrangement of atoms. This interaction is key to understanding their nature.
How Do Chemists Define Acids?
Chemists, naturally, have a more precise way of talking about acids than just taste or color changes. One of the main definitions you'll hear is that an acid is a substance that gives away protons, or what we sometimes call hydrogen ions. Think of it like this: the acid has these little hydrogen bits with a positive charge, and it's ready to share them with another molecule. This sharing, or donating, is a core part of how many acids behave in solutions, pretty much.
Another important way to think about acids is that they are substances that can accept a pair of electrons. This might sound a bit technical, but basically, some acids are looking for a pair of electrons to bond with. So, they act like a receiver in a chemical exchange. This definition broadens our understanding of what an acid can be, showing that it's not just about giving away hydrogen but also about how it interacts with electrons. It's a different angle, you know, but just as valid.
Proton Donors and Electron Acceptors - The Science Behind the Acid Bath Murderer's Tool
It's interesting to note that just because a compound has hydrogen in it doesn't automatically make it an acid. For instance, water has hydrogen, but it's not typically considered an acid in the same way that, say, vinegar is. The hydrogen has to be in a specific arrangement or be capable of being released in a certain way for the substance to truly act as an acid. It's a bit more nuanced than just looking at the chemical formula, you see.
There are, in fact, a couple of main ways chemists look at acids today. These different perspectives help us understand the wide variety of substances that fall under the "acid" umbrella. Whether it's about donating protons or accepting electrons, these definitions provide a framework for categorizing and predicting how these compounds will react. It's like having different lenses to view the same chemical phenomenon, which is pretty cool, actually.
Is That Acid Strong or Weak?
When we talk about acids, it's not a one-size-fits-all situation; they come in different strengths. Some acids are what we call "strong" acids. Think of something like hydrochloric acid, which you might find in some industrial settings or even in your own stomach, helping with digestion. These strong acids are very good at giving away their protons, and they do it almost completely when they're in a water solution. They're pretty potent, in a way.
On the other hand, we have "weak" acids. Acetic acid, the stuff that gives vinegar its distinctive taste and smell, is a great example of a weak acid. Unlike strong acids, weak acids don't give away all of their protons when they're in water. They only release some of them, making them less reactive and generally safer to handle in everyday situations. It's a matter of degree, really, how much they "ionize" in water.
Hydrochloric vs. Acetic - Different Strengths for the Acid Bath Murderer's Concept
The strength of an acid is pretty important because it tells us a lot about how it will behave. A strong acid will react much more vigorously and completely than a weak acid. This difference in strength is tied to how readily the acid donates its hydrogen ions or accepts electrons. So, while both are acids, their power and the way they interact with other substances can be quite different. It's a key distinction for chemists, obviously.
This idea of strength helps us understand why some acids are used for very powerful industrial processes, while others are safe enough to put on our food. It's all about that chemical willingness to participate in reactions. A strong acid is always ready to go, so to speak, while a weak acid is a bit more reserved in its reactivity. You know, it's a spectrum, not just an either/or situation.
Where Do We Find Acids in Daily Life?
It might surprise you how common acids and their counterparts, alkalis (or bases), are in our daily lives. They're not just found in science labs or scary headlines. For example, right in your own home, you'll find acids in things like cleaning products, citrus fruits, and even some sodas. They're pretty much everywhere, if you start looking for them.
Think about our own bodies; we have acids working hard inside us. Stomach acid, for instance, is a very strong acid that helps us break down food. And then, there are acids in car batteries, which are essential for getting your vehicle to start. So, they play a pretty big role in both biological processes and industrial applications, as a matter of fact.
From Batteries to Bodies - The Ubiquitous Nature of the Acid Bath Murderer's Material
In industry, acids are used for all sorts of things, from manufacturing fertilizers to refining metals. They are truly versatile chemical tools. And, of course, in school science labs, acids are fundamental for teaching students about chemical reactions and properties. They're a basic part of understanding how the world works at a molecular level, you know.
One of the jobs of a chemist is to tell the difference between all these substances, to classify them, and to understand how they will behave. This means knowing whether something is an acid, a base, or something else entirely. It's about being able to predict their interactions and use them safely and effectively. It's a bit like being a detective, in some respects, figuring out the identity of chemical compounds.
What Happens When Acids Meet Bases?
We touched on this earlier, but it's worth a closer look: what happens when an acid meets a base? This interaction is one of the most fundamental chemical reactions you can observe. When a certain amount of an acid is added to the same amount of a base, something pretty cool happens. They essentially cancel each other out, or "neutralize" each other, as chemists would say. It's a very precise kind of balancing act.
The result of this neutralization reaction is usually the creation of water and a salt. This is why, for example, if you have an upset stomach from too much acid, you might take an antacid, which is a base. The antacid works by neutralizing the excess acid in your stomach, bringing things back to a more balanced state. It's a practical application of this basic chemical principle, you know, in daily life.
Neutralizing the Threat - Counteracting the Acid Bath Murderer's Chemical Action
The formation of water and a salt is a pretty neat trick. It shows how two substances, which might individually be quite reactive or even dangerous, can combine to form something much less so. This process is essential in many industrial applications, like wastewater treatment, where acids or bases need to be neutralized before they can be safely released. It's about maintaining a kind of chemical harmony, basically.
So, an acid is considered the opposite of a base, and their interaction is a cornerstone of chemistry. Understanding this relationship helps us grasp how various compounds behave in different environments and how we can control their reactions. It's a pretty powerful concept, actually, that has a lot of real-world implications, from medicine to environmental science.
What About pH and Litmus Paper?
When we talk about acids, we absolutely have to talk about pH. The pH scale is a way of measuring how acidic or basic a solution is. Acids have a pH value that is less than 7. The lower the number, the stronger the acid. So, something with a pH of 0 or 1 is very, very acidic, while something with a pH of 6 is only slightly acidic. It's a simple number system that tells you a lot about a substance's nature, you know.
And then there's that litmus paper again. We mentioned that acids turn blue litmus paper red. This is a very visual indicator of acidity. If you have a solution and you're not sure if it's an acid, a quick dip with blue litmus paper can give you an immediate answer. It's a straightforward test that has been used for a very long time to identify acids, pretty much universally.
The pH Scale and Red Litmus - Visualizing the Acid Bath Murderer's Impact
The pH scale ranges from 0 to 14. A pH of 7 is considered neutral, like pure water. Anything above 7 is basic or alkaline. So, acids occupy the lower end of this scale. This numerical representation gives chemists a precise way to compare the strengths of different acids and bases. It's a universal language for acidity, which is pretty handy, as a matter of fact.
These tools, the pH scale and litmus paper, are fundamental for anyone working with chemicals, or even just trying to understand the chemistry around them. They provide quick and easy ways to assess the acidic nature of a substance, which is essential for safety and for carrying out specific chemical processes. They're basic but powerful, you know, for understanding chemical properties.
Can Acids React with Metals?
Yes, acids can certainly react with some metals, and this is a pretty interesting characteristic. When certain acids come into contact with specific metals, a chemical reaction takes place where hydrogen gas is released. You might see bubbles forming on the surface of the metal as the gas escapes. This is a clear sign that a reaction is happening, and the acid is, in a way, dissolving the metal over time.
This reaction is why you wouldn't store strong acids in containers made of reactive metals. The acid would simply eat through the container, causing all sorts of problems. It's a powerful demonstration of the acid's ability to interact with and change other substances. This property is also used in various industrial processes, like cleaning metal surfaces or in the production of certain chemicals. It's a pretty important interaction, honestly.
Releasing Hydrogen - The Destructive Power in the Acid Bath Murderer's Scenario
The ability of acids to react with and sometimes dissolve other substances is one of their defining features. This isn't just limited to metals; acids can react with many different kinds of materials, depending on their strength and the nature of the other substance. It's a testament to their chemical activity and their capacity to bring about change at a molecular level. You know, it's a fundamental aspect of their character.
So, when you think about the phrase "acid bath," this reactive quality of acids is probably what comes to mind. It's the idea that these substances can break down and transform materials through chemical processes. This property is what makes acids so useful in some contexts, but also potentially dangerous if not handled with proper care and understanding. It's a pretty strong force, in some respects.
Why Are Acids So Variable?
In life, acids can be incredibly varied in their form and how they behave. They aren't just one single type of chemical. Some are liquids, like the ones we've mostly discussed, but some can also be solids or even gases, though we usually encounter them dissolved in water. This variety means that their uses and their potential impacts can be really, really different depending on their specific chemical makeup. It's a broad category, basically.
The meaning of "acid" itself, as we've seen, can be understood in a few different ways by chemists. Whether it's about tasting sour, donating protons, or accepting electrons, these definitions help us categorize a wide range of substances that share some fundamental chemical behaviors. It's about having different frameworks to grasp the whole picture of what an acid is and does, you know, in the chemical world.
Forms and Functions - The Diverse Properties Relevant to the Acid Bath Murderer
In simple terms, acids are substances that have a sour taste and can make blue litmus paper turn red, showing their acidic nature. They're also known for their ability to react with bases to form new compounds. These are the very basic characteristics that most people can grasp, even without a deep science background. It's a pretty straightforward way to start thinking about them.
But beyond these simple ideas, the world of acids is quite complex. They can be extremely powerful or very gentle, depending on their concentration and chemical structure. This variability is what makes them so useful in countless applications, from the food we eat to the products we use every day. It's a fascinating area of chemistry, honestly, with so much to explore and understand about how these substances shape our physical world.
- Ujjwal Reddy Twitter
- Toxicity Twitter
- %C3%B8%C3%BA%C3%B8%C3%BB%C5%93 %C3%B8%C3%B9%CB%86%C3%B9%C3%B8%C3%B9%C3%B8%C3%BB%C5%93
- Mattbegreat Twitter
- Reya Sunshine Twitter